Without a doubt the work done by the pharmaceutical, biotech, clinical research, drug development industry and the Food and Drug Administration is worthy of this distinction for 2020. What was accomplished is just short of miraculous.
The federal government (FDA) and the corporations cut no corners. I have no doubt that everything was done according to established good clinical principles. What was done was the speeding up of the regulatory processes; decreasing the workflow processing time. Turn around time on data review and decision making was the focus. The researchers and the reviewers of the data had Covid-19 treatments and vaccines at the forefront of their priorities.
The typical time for vaccine development to get approved is 4 years. The first two approved were done within 12 months!! There are several more in development.
We are now beginning to see the impact the vaccine is having in the decline of daily new cases. Many people have now received their second dose.
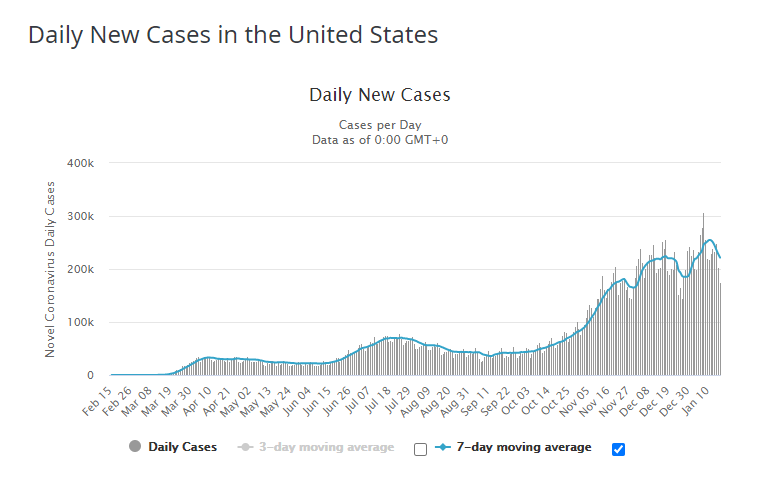
The United Kingdom began vaccinating their population one week earlier than the US and you can see the impact to their daily new cases as well.

The results of such rigorous work to develop a vaccine that helps prevent the spread of this serious and clearly life-threatening disease in an expedited timeframe is true evidence of scientific innovation and public and private collaboration worldwide.