
GLSA offers global reach for clinical trials with our network of Clinical Research Organizations (CRO’s). This article discusses the advantages of running trials in the Philippines. Our partner in the Philippines brings you geographic diversity and full service capabilities. Clinical Trial Management, Site Management and Trial Support Services are just the beginning of why the Philippines is emerging as a sweet spot for running trials.
We are excited to be partnered with this CRO. They are uniquely positioned to provide diversity and expertise for XUS trials.
Why The Philippines
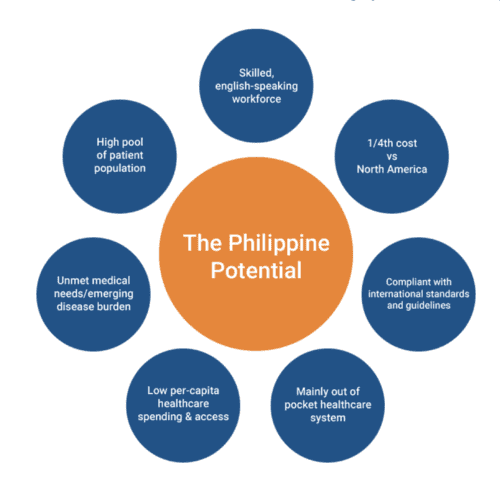
The Philippines offers a sweet spot for Clinical Research for more than a few reasons.
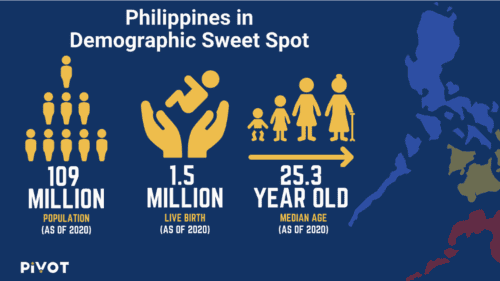
The large population of 109M means a large pool of potential candidates. The quality of patients is further expanded due to a very high birth rate, opening up opportunity for pediatric trials.
In addition, the mainly “out of pocket” healthcare system has resulted in a significant population of Filipinos who have not experienced drugs taken by individuals in countries with more advanced healthcare systems. This represents opportunity for patient naive trials and clinical trials in general.
The regulatory climate in the Philippines also presents distinct advantages. Recent improvements allow that studies can begin in 60 days, sometimes 30. In addition, there are over 46 Ethics Review boards (ERB’s) ready to review and approve clinical trial protocols.
The Philippine advantage for XUS trials is evident. Our partner is ready to explore what’s possible with you. You can learn more here Clinical Trials in the Philippines.
Now let’s explore the expertise this partner offers you
In the Philippines, your protocols can be approved quickly. Our partner manages clinical trials from Phase I to IV. Their team of experts have extensive experience in many therapeutic areas including Oncology, Vaccine, Infectious Diseases and the large treatment naive population pool offers unique recruitment advantages. The services include:
- Clinical Trial Management
- Post Marketing Surveillance (PMS) and Real World Evidence (RWE)
- Site Management
- Clinical Trial Support Services

Clinical Trial Management
Managing clinical trials comes with many complex challenges. Our partner has developed a comprehensive solution to manage the entire process. This Drug and Device Trial Management for Clinical Trials solution will streamline and automate the process making it easier to achieve your desired outcomes.
Post Marketing Surveillance (PMS) and Real World Evidence (RWE)
Safety and efficacy are always of primary concern. You can be assured that products are continually monitored for adverse reactions and evaluated through a filter of RWE to ensure effectiveness in the real world. GLSA’s partner provides end to end support from pre-regulatory and start-up to close-out and clinical study analysis reporting.
Site Management
The SMO platform provides sites and Principal Investigators with excellent clinical trial related services.
Capabilities
- Contract negotiations
- Submission for Institutional Review Board or Independent Ethics Committee (IRB/IEC) approval*
- Patient counseling
- Patient recruitment and retention
- Patient follow-up
- Informed Consent Form (ICF) translation into vernacular languages*
- Site initiation and trial close-out operations
- Trial-related documents archival and maintenance
- Reporting serious adverse events to the Sponsor or CRO and the IRB/IEC
- Ensuring protocol compliance
- Advising and alerting investigators of potential protocol violations
- Advising and alerting investigators of potential ICH-GCP violations
- International site accreditation and training
- Networking of research sites
Clinical Trial Support Services
Pharmacovigilance is an integral part of the drug development process and in the Philippines you are working with a partner who provides end-to-end project management to support PV efforts. You can be assured that your PV program meets the highest standards of safety, effectiveness.
The advantages for you to run your trials in the Philippines are clear and the capabilities of this CRO are a full spectrum offering. Learn more about the possibilities right here Clinical Trials in the Philippines.