So, it finally happened, on that glorious day which shall long be remembered, these minutes will be heretofore submitted to the USA Library of Congress, the Smithsonian Institute and for reasons unknown, to the Sydney Opera House on the northside bulletin board for public postings.
On May the 20, in the year 2021 of the Gregorian calendar, it was noted that the GLSA (Global Life Sciences Alliance) and FOCM (Friends of Chris Matheus) Networking organization did hold an online (virtual) networking event. The meticulously planned event went terribly awry when but half of the positive RSVPs failed to show up. That said, it was a resounding success for the initial such event. A total of 21 attended.
The meeting started off with an acknowledgement that it was Global Clinical Trials Day and a toast was given to the clinical research industry for saving the world from Covid-19 and to James Lind, the Scottish doctor who initiated the first controlled randomized clinical trial on May 20, 1747 aboard a sailing ship. Dr. Lind divided twelve sailors sick with scurvy into six groups of two. They all received the same diet but, in addition, each group was given a different treatment. Only the two sailors who received citrus fruits improved and returned to work.
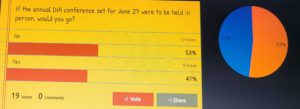
Chris then introduced the GLSA members to the FOCM community. After a bit of general discussion, several polls were taken. About half of the group is reluctant to resume conference travel immediately, preferring to wait a few more months. Slightly more than half have been vaccinated or acquired immunity through catching the virus. An interesting opinion was voiced that perhaps as members of the clinical research industry, we should set the example by all being vaccinated. I, for one and I believe I speak for many of the others have the utmost confidence that not a step was missed, not a shortcut taken in the development of the available vaccines. Given the prioritization and urgency of vaccine development, we were able to speed up the data review process. The one thing that the sped up development lacked is longer term safety and side effect data. However, vaccine side effects rarely (I can’t think of any) change the longer from the time of injection.
Then it was time for speed networking! The assertion has been made by Chris that each of us in the clinical research industry are within 2 degrees of separation from each other. We had 4 different sessions. Attendees were randomly put into different “rooms” with the assignment to each introduce themselves to the group, sharing where they’d worked the previous 10-20 years and what they’re doing now to see if they could identify who they knew in common. Good information was exchanged and several new connections were made which can improve the management of clinical trials.

Join us in our next event! – June 16.
Event Attendees:
David Holland, Cmed Research
Jon Matheus, Pancrazi Real Estate
Sheila Mahoney-Jewels, Life Science Hub
Eric Nier, Block Clinical
Lynne Becker, Power of Patients
Nadia Bracken, Medidata
Christine Ver Straate, GLSA
Mitchell Efros, Verified Clinical Trials
Cassandra Hui, HealMary
Denise McNerney, GLSA
Joe Buser, GLSA
Tom Ryan, GLSA
Kalyan Ghosh, Inference Inc
Marty Frazier, GLSA
Tanusree Bhattacharyya, Inference Inc
Zulma Varela, GLSA
Mike O’gorman, Life Science Marketplace
David Gibboni, DJGibboni Consulting
Eric Mayer, EDP Biotech
Craig Fernandes, EDP Biotech